

Treponema pallidum (TP) is a slender, spiral-shaped Gram-negative bacterium belonging to the genus Treponema within the family Spirochaetaceae. This microorganism is the causative agent of syphilis, primarily transmitted through sexual contact, blood, or vertical transmission from mother to child. TP is highly invasive, capable of infecting various human tissues and organs. However, its cultivation in vitro is extremely challenging, and detection and research have long relied on serological methods. The World Health Organization (WHO) has classified syphilis as a globally prioritized sexually transmitted infection for prevention and control. Continuous epidemiological surveillance and pathogen research are essential for understanding its transmission mechanisms and improving control strategies.
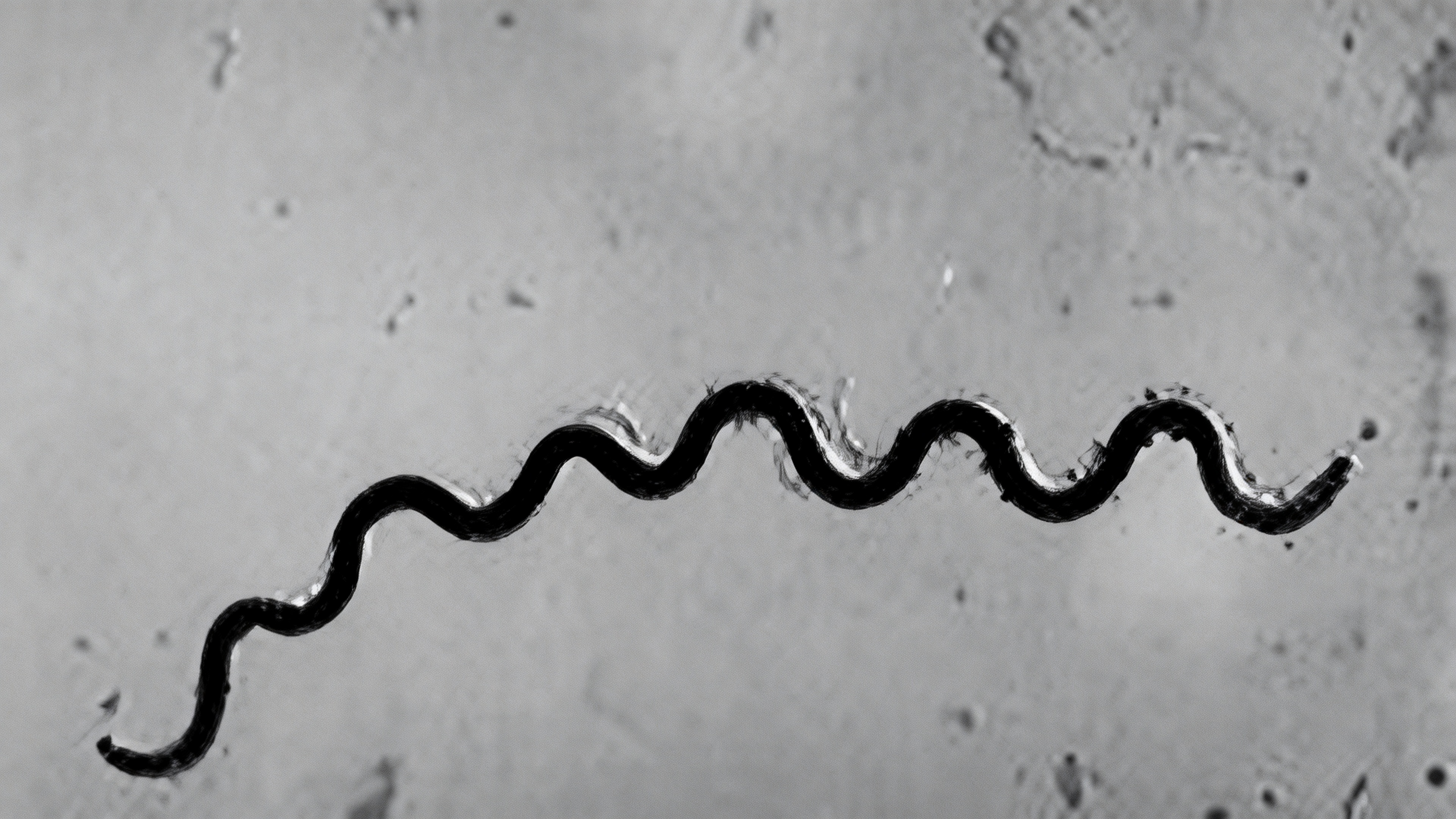
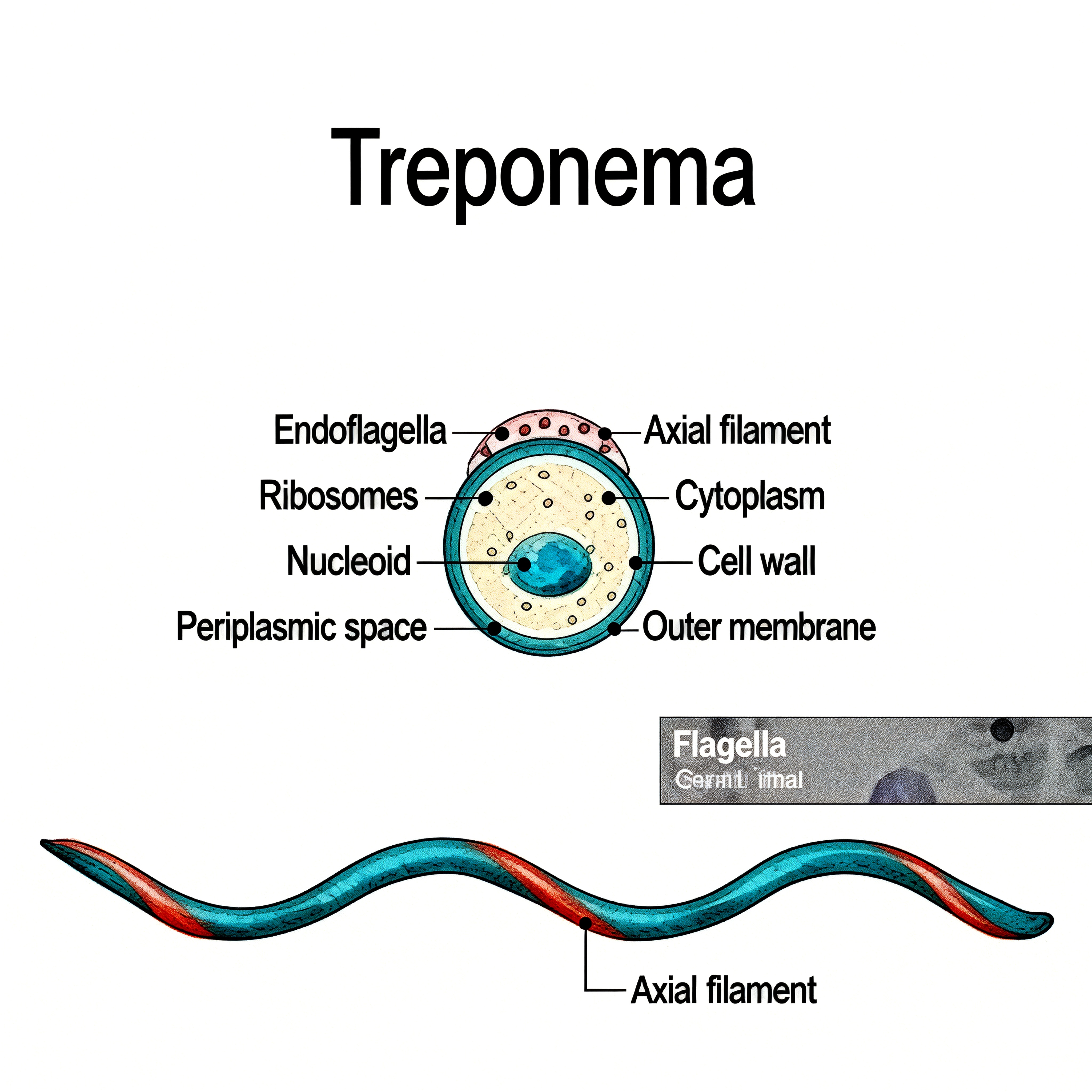
Treponema pallidum belongs to the family Spirochaetaceae, and its cells exhibit a slender spiral shape, measuring approximately 6–15 µm in length and 0.1–0.2 µm in diameter, with 8–14 regular and tightly coiled spirals. TP is a Gram-negative bacterium, but its staining properties are atypical due to its low outer membrane lipid content and lack of typical lipopolysaccharides. The entire bacterial cell is structured from the inside out, primarily comprising the protoplasmic cylinder, periplasmic flagella, and outer membrane. The protoplasmic cylinder contains fundamental cellular structures such as the cytoplasm, nucleoid region, and ribosomes, serving as the center for metabolism and synthesis. The periplasmic flagella are located in the periplasmic space between the outer membrane and the cell wall, with 3–4 flagella attached at each end. They drive the characteristic bending and rotational movement of the bacterium, playing a key role in its invasiveness and dissemination. The outer membrane is composed of lipoproteins, transmembrane proteins, and a small amount of lipids. Among these, abundant transmembrane proteins (such as the Tpr family proteins) are exposed on the surface and may be involved in processes like host cell attachment and immune evasion. The limited antigenic targets on the outer membrane (such as TpN47, TpN17, and TpN15) are the primary recognition sites for serological detection. However, the low expression levels and variability of TP outer membrane proteins present ongoing challenges for the development of related detection methods and vaccines.
Our company provides a comprehensive range of antigens and antibodies for Treponema pallidum.
【Antigens】
| Product Name | Catalog# | Contact |
| NebuSelect™ Recombinant Treponema pallidum FlaB3 Protein, 1-285aa, His-tag, SUMO-tag | NBL-292591 | ☎ Technical Support >> |
| NebuSelect™ Recombinant Treponema pallidum/TP tmpA Protein, 21-345aa, His-tag | NBL-292592 | ☎ Technical Support >> |
| NebuSelect™ Recombinant Treponema pallidum/TP TPF1/Antigen 4D Protein, 1-177aa, His-tag | NBL-292593 | ☎ Technical Support >> |
| NebuSelect™ Recombinant Treponema pallidum/TP TPP15 & tmpA & TPP47 Protein, TPP15 19-141aa & tempA 20-345aa & TPP47 21-434aa, His-tag | NBL-292594 | ☎ Technical Support >> |
| NebuSelect™ Recombinant Treponema pallidum/TP TPP17/17 kDa lipoprotein Protein, 22-156aa, GST-tag | NBL-292595 | ☎ Technical Support >> |
| NebuSelect™ Recombinant Treponema pallidum/TP TPP47 Protein, 20-434aa, His-tag | NBL-292596 | ☎ Technical Support >> |
【Antibodies】
| Product Name | Catalog# | Contact |
| NebuSelect™ Anti-Treponema pallidum FlaB3 Polyclonal Antibody | NBAB-194433 | ☎ Technical Support >> |
| NebuSelect™ Anti-Treponema pallidum tmpA Polyclonal Antibody | NBAB-194434 | ☎ Technical Support >> |
| NebuSelect™ Anti-Treponema pallidum TPP47 Polyclonal Antibody | NBAB-194435 | ☎ Technical Support >> |
| NebuSelect™ Anti-Treponema pallidum TPP17 Polyclonal Antibody | NBAB-194436 | ☎ Technical Support >> |
| NebuSelect™ Anti-Treponema pallidum TPF1/Antigen 4D Polyclonal Antibody | NBAB-194493 | ☎ Technical Support >> |